TAEUS®
The future of TAEUS®
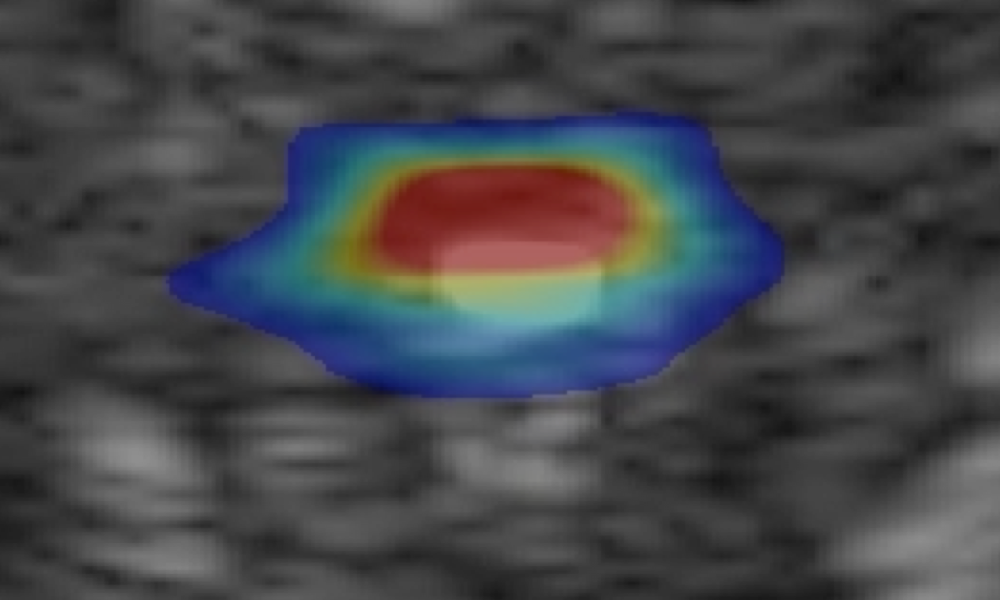
TAEUS® will initially focus on tissue composition, quantifying fat in the liver to help clinicians identify a pervasive and costly disease.
But TAEUS®’ potential reaches far beyond just a single application.
In the future, we believe that TAEUS® will visualize tissue temperature and function, helping guide procedures that treat conditions such as cancer and atrial fibrillation, or help identify arterial plaque and internal bleeding.

Tissue composition
Quantify fat vs. lean tissue to detect Non-Alcoholic Fatty Liver Disease (NAFLD).
Learn more >
Tissue temperature
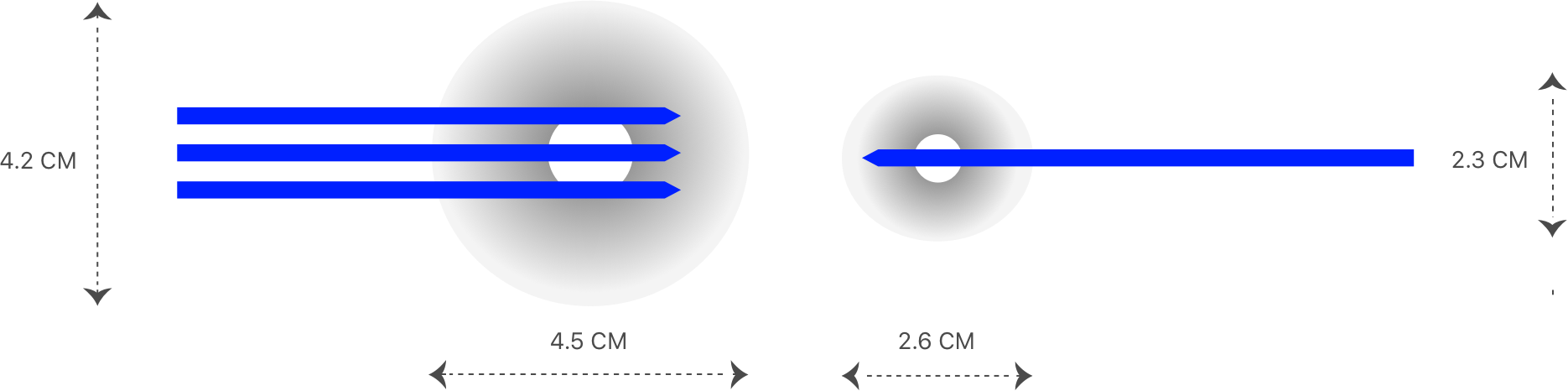
Display tissue temperature in real time, facilitating more precise application of heat or cold during energy-based surgery.
Learn more >What is TAEUS®?
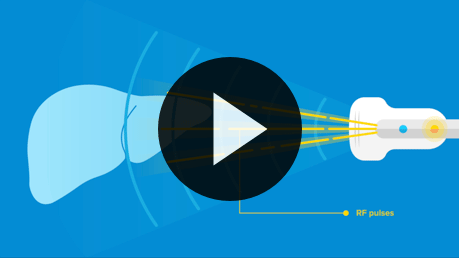
Thermo-Acoustic Enhanced UltraSound will revolutionize what doctors can see and do at the patient bedside.
Watch the video >