Read the latest
Find out what’s new with ENDRA, TAEUS® and the future of medical imaging.
We’re changing the way clinicians measure fat in the liver, making it easier, less expensive, and more accessible for everyone.
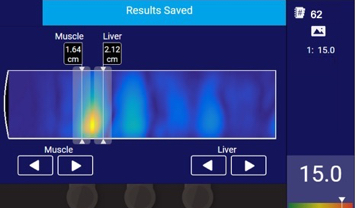

Thermo-Acoustic Enhanced UltraSound® (TAEUS®) combines radio frequency and ultrasound waves to measure fat in the liver in a groundbreaking way.

It is CE-marked and commercially available in Europe, and pairs seamlessly with any B-mode ultrasound machine.
Learn how ENDRA is using ultrasound to help make difficult-to-diagnose diseases easier to find and treat.
WATCH THE VIDEO >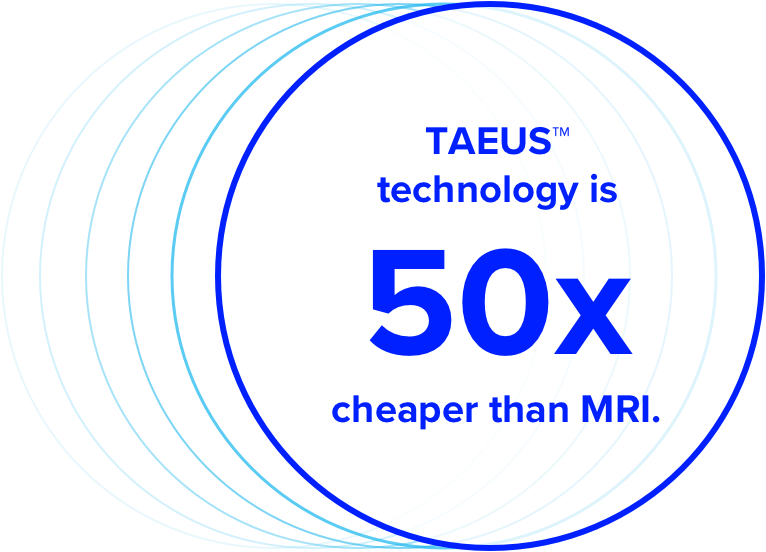
TAEUS® fits in seamlessly with an ultrasound suite—small footprint, similar workflow.
The technology is non-invasive, unlike liver biopsy, and 50x cheaper than MRI. TAEUS® allows clinicians to measure steatosis in less than a minute—at the patient point-of-care.
TAEUS® connects to conventional ultrasound to measure permittivity. Radio frequencies (RF) stimulate tissue and create small sonic waves that are picked up and decoded by our proprietary algorithms.
ENDRA is the first to use the unique combination of RF and ultrasound to produce meaningful data quickly, efficiently and cost-effectively.
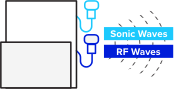
Locate the region for fatty liver tissue assessment with any traditional B-mode ultrasound.
Pulse radio frequency (RF) waves into target tissue with TAEUS®.
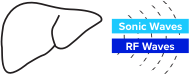
TAEUS® RF energy creates small sonic waves that represent a unique signature for lean & fat tissues.

TAEUS® sonic waves are detected by separate TAEUS® ultrasound receiver.
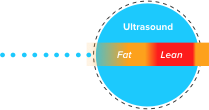
TAEUS® outputs include ultrasound image overlays or quantitative data translated into clinically useful metrics.
“ENDRA’s TAEUS® technology could be a game-changer for the clinical care cycle of liver [and other] disease—from screening to diagnosis to therapy guidance.”
Find out what’s new with ENDRA, TAEUS® and the future of medical imaging.
ENDRA CLINICAL ABSTRACT: EUROPEAN ASSOCIATION FOR THE STUDY OF THE LIVER (EASL), STEATOTIC LIVER DISEASE (SLD) SUMMIT, SEPT 2023
ENDRA CLINICAL ABSTRACT: EUROPEAN ASSOCIATION FOR THE STUDY OF THE LIVER (EASL), INTERNATIONAL LIVER CONGRESS, JUNE 2023

As one of the world’s foremost healthcare technology companies, and the global leader in medical imaging, GE understands the importance of broadening access to better healthcare.

ENDRA operates a Quality Management System which complies with the requirements of ISO 13485:2016 & EN ISO 13485:2016 for the following scope:
Certificate number: MD 697226
Effective Date: 2025-04-02
Expiry Date: 2028-04-01